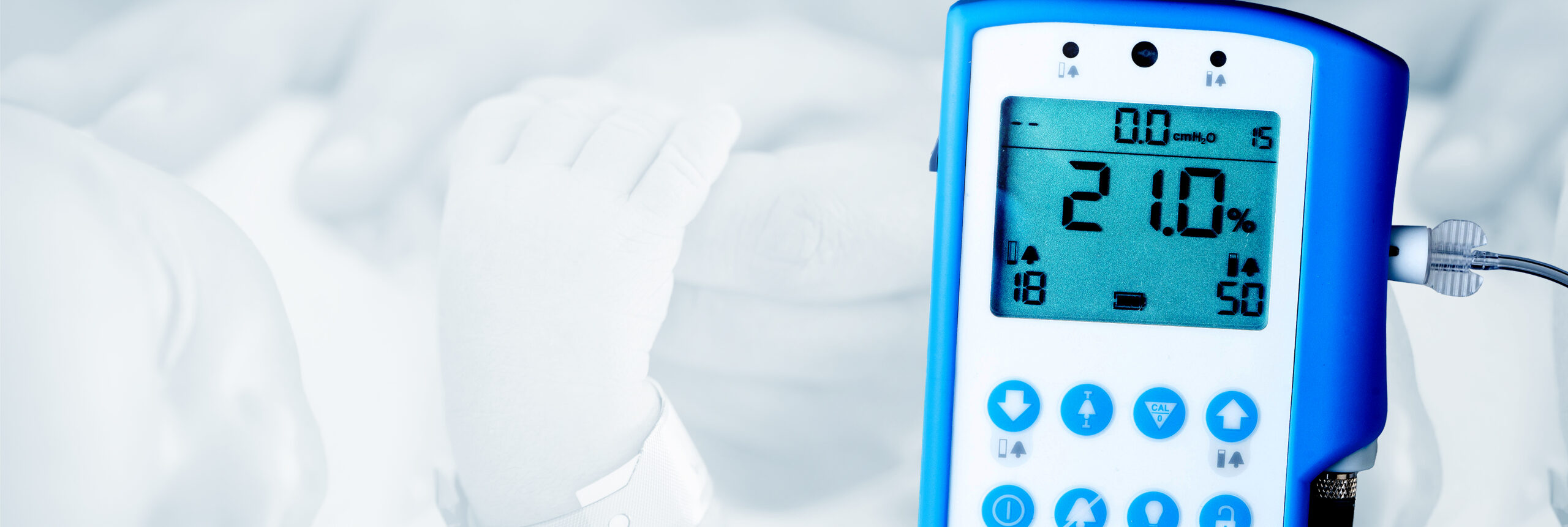
Salt Lake City, UT – Maxtec, a global leading manufacturer of respiratory care products, is excited to announce the launch of its newest device, the MaxO2 ME+p. This device is an oxygen monitor with integrated pressure monitoring intended for continuous monitoring of the concentration of oxygen and pressure being delivered to patients ranging from newborns to adults. This device will help provide clinicians with the critical data they need to aid the delivery of effective care to patients.
The MaxO2 ME+p is a state-of-the-art device that helps healthcare providers monitor the oxygen concentration and pressure being delivered to patients. In a NICU setting, it can serve as a useful tool to integrate into a bubble CPAP therapy setup. By providing real-time, accurate data on oxygen concentration and pressure, the MaxO2 ME+p helps healthcare providers ensure that their patients are receiving the correct therapy.
“We are thrilled to introduce the MaxO2 ME+p to the market,” said Kathy Ouellette, President and CEO of Perma Pure Group (consisting of Maxtec and Perma Pure). “This device is the result of our ongoing commitment to developing innovative medical devices that make a real difference in the lives of patients and healthcare providers. We believe that the MaxO2 ME+p is a game-changer in the field, and we are excited to see the impact it will have on patient care.”
Maxtec is dedicated to providing clinicians with the tools they need to deliver effective care to their patients. The MaxO2 ME+p is the latest example of this commitment, and Maxtec is proud to bring this product to market as they continue to pursue a mission of helping the world to Breathe Easier and Be Healthier.
For more information about the MaxO2 ME+p, please visit maxtec.com or contact us directly at (385) 549-8000.
View full press release here: